
Photomicrograph depicting the siliceous frustules of fifty species of diatoms arranged within a circular shape. Diatoms form the base of many marine and aquatic food chains and upon death, their glassy frustules form sediments known as diatomaceous earth (Credit: Randolph Femmer / USGS, Public Domain)
Plankton recorders have been used for field research dating all the way back to the 1930s and yet, there is still no perfect solution to quantifying plankton community structures and dynamics in aquatic ecosystems.1 Zooplankton and phytoplankton play critical roles in a lake system’s food web as well as being important for various ecological functions.
The categories of Zooplankton and phytoplankton are also wide-branching, containing a multitude of organisms varying in size, behavior, appearance and other factors that make tracking and understanding the microbial food web difficult. In the past, the most common method used to count and classify heterotrophic organisms was manually through a microscope. However, this process is time-consuming and doesn’t allow for large data pools to be gathered consistently.
While recent developments in the field have allowed for underwater cameras, flow-through cuvettes and holographic flow cytometry to rise in popularity for monitoring microorganisms, these systems are not much more effective. While they decrease the labor needed for individual sampling, these methods are all “based on acquiring static images of plankton and then subsequently measuring and classifying them either manually or automatically by machine recognition.” 1 Ultimately, the goal of this research was to test how effective and accurate automated measuring and classifying software could be. If successful, these automated systems would save a great deal of time, money, and resources on studies—virtually allowing them to run in the background while more time is spent assessing the data.
Methods – Testing Automated Systems
The automated method proposed in this ASLO study involved the use of video cameras and fluorescent imaging in a mesocosm environment. During the study, two video cameras were focused on different optical chambers of different sizes. The chambers were then filled and emptied repeatedly by synchronized pumps. Imaging is then transported to a computer that conducts real-time motion analysis on the respective video feeds.
In order to test the accuracy of the automated system, “Results were validated against Chl a measurements and microscopy counts.” 1 The study ultimately assessed the following parameters: organism size, swimming velocity, motility patterns, and chlorophyll fluorescence density. The automated system allows scientists to collect data on swim patterns, something not as easily gathered in manual counting assessments.
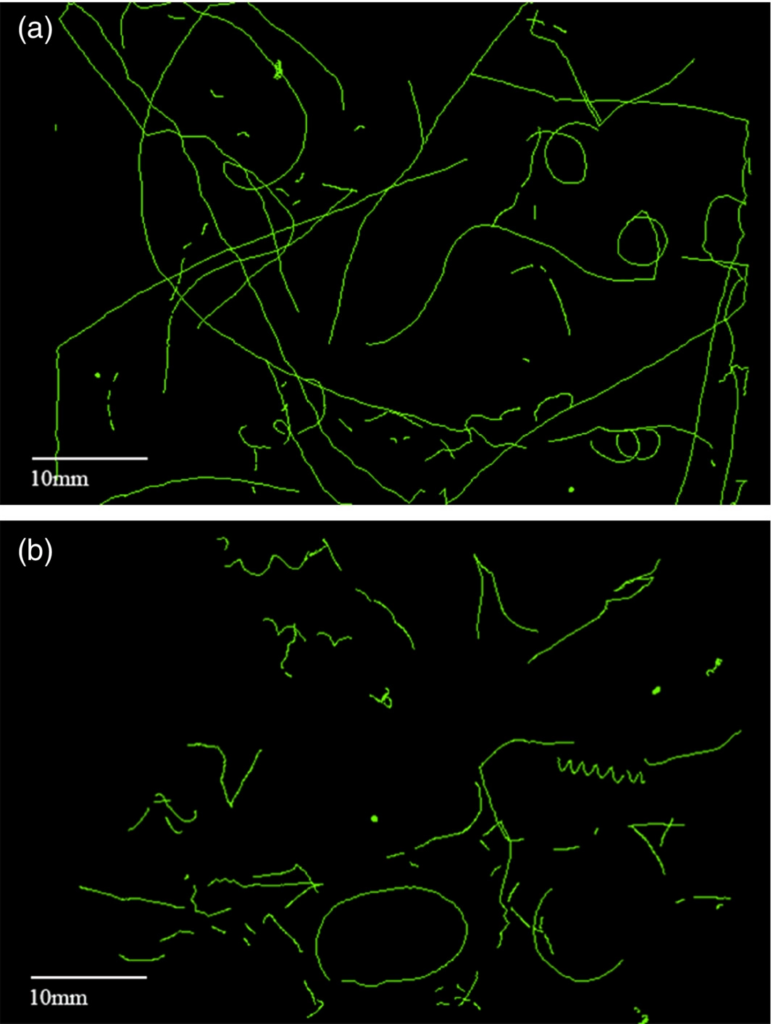
All accumulated tracks from the two time intervals of lights on (8:00 h to 20:00 h) and lights off (20:00 h to 8:00 h). (a) Lights on period. (b) Lights off period (Blackburn et al.,2022).
Results
In general, the proposed automated method worked just as well as more traditional means of quantifying microorganism communities. When observing phytoplankton, there were some differences spotted between adjacent fractions. This variation was attributed to the following:
- “The automatic counter only seeing chlorophyll in purely autotrophic cells, the extent of which can be smaller than the cell itself;
- Cells in colonies may be concatenated into one larger entity;
- Larger cells with distributed chloroplasts can be split into smaller sized entities.” 1
Even with the variations and difficulties presented, the researchers were not deterred from their mission to understand these organisms. Stating, “It is a goal and a challenge to understand more about this largely invisible world and the niches that exist for survival and fitness optimization.”2 In the end, this technology has a place in researching microorganisms, even if it isn’t perfect. Instead, they recommend using these automated systems to measure the dynamic properties of ecosystems across species and groups.
Sources
- Blackburn, N., Haecky, P., Jurgensone, I., Griniene, E., Brugel, S., Andersson, A. and Carstensen, J. (2022), The use of an automated organism tracking microscope in mesocosm experiments. Limnol Oceanogr Methods. https://doi.org/10.1002/lom3.10521
- Stocker, R. 2012. Marine microbes see a sea of gradients. Science 338: 628–633. doi:10.1126/science.1208929







